Feed water
|
||||||||||||||||||||||||||||||||||||||||
IntroductionIon exchange resins exchange ions. Not a surprise, but the composition of the feed water affects plant performance. It is therefore essential to know precisely the water composition of the feed to the ion exchange system. The following components and characteristics should be known:
We will examine the effect of all above parameters and try to set practical limits for each. |
||||||||||||||||||||||||||||||||||||||||
Salinity (water analysis)This is the single most important item to estimate the performance of an ion exchange system. It is also one of the first things to check when plant performance deteriorates. You cannot rely on an analysis that was made months or years ago. Some effects of a change in salinity are:
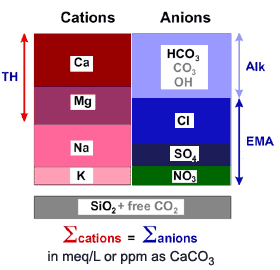
The picture below is a schematic representation of a water analysis, with cations and anions. A good water analysis must be balanced. See also a detailed description of the water analysis, with the concentration units to use and a table of the most common ions in water. If the water analysis varies according to season, plant performance should be re-assessed, and perhaps operating conditions re-adjusted, to reflect the seasonal variations. If you don't analyse the water yourself, give a sample to a reputable laboratory for testing. If your feed water is city water, you should be able to obtain an accurate analysis from your municipality. When re-assessing the performance of a plant, or optimising it, it is recommended to use the most probable analysis for the basic calculation, then to re-run the calculation with seasonal analyses to estimate plant throughput under various conditions. All the water analyses should be real, not maxima, averages or minima. We strongly recommend that you should update the expected performance of the plant based on actual operating conditions. You should collect the necessary data:
Salinity limits
Ion exchange is the perfect technology for low concentrations. At high salinity, the cycles become very short, regenerant consumption increases and in extreme cases the water required for regeneration may exceed the volume of treated water. As a guideline, a salinity of 20 meq/L (1000 ppm as CaCO3) seems to be the high limit, with some exceptions. Higher salinity water is probably best treated with RO. Sea water cannot be demineralised by ion exchange, as the resins would be exhausted in less than 3 bed volumes. |
||||||||||||||||||||||||||||||||||||||||
Suspended solids and turbidityIdeally, the feed water to an ion exchange vessel should be perfectly clear and free of suspended solids. It is essential to ensure that mechanical filters installed ahead of an ion exchange system operate properly. Insufficient filtration resulting in excessive suspended solids may cause:
Suspended solids are traditionally measured by filtration on a 0.45 µm filter and expressed as dry mass. The tolerated amount of suspended solids varies according to the ion exchange technology and to the run length. If the resins can be easily backwashed and cleaned, a higher quantity of suspended solids is acceptable.
Limits for suspended solids
There is no simple number here: the most sensible way is to calculate the load of solids during one cycle and to express the result per square metre of vessel (cross-section). Here some suggestions:
Turbidity limits
Turbidity is not used much in conjunction with ion exchange systems. See suspended solids above. For floating bed systems without a backwash tower, it was found that 1 NTU is more than what the columns can tolerate. |
||||||||||||||||||||||||||||||||||||||||
TemperatureThe temperature of the feed water (and of the regenerants) can affect plant performance.
Temperature limits
See the table with limits of temperature for all anion exchange resins.
|
||||||||||||||||||||||||||||||||||||||||
pH valueIon exchange resins can tolerate any pH value (0 to 14) without suffering damage, provided strong osmotic shocks due to rapid change of pH or concentration are avoided. In service however, resins operate only within pH limits: cation resins cannot operate at very low pH, or anion resins at very high pH, because they would be permanently regenerated and unable to exchange other ions. Similarly, the resins are normally not used in very concentrated solutions. This is why in practice the table below should only go up to pH 12 and down to pH 2, which would be 10 meq/L of NaOH or acid respectively. pH limits
|
||||||||||||||||||||||||||||||||||||||||
OrganicsOrganic matter in water can interfere with ion exchange. The main effect of organics is irreversible fouling of anion exchange resins.
The traditional measurement of organics (COD) in natural water uses the potassium permanganate oxidation method, and its result is expressed in mg/L as KMnO4. Unfortunately, there is no direct correlation between this method and the more modern analysis of TOC (Total Organic Carbon). However, experience has shown that as a rule of thumb, 1 mg/L TOC (1 ppm as C) can be roughly translated into 5.5 mg/L (5.5 ppm) as KMnO4. Limits of organic load
See the table for all anion exchange resins (same as temperature table). |
||||||||||||||||||||||||||||||||||||||||
Other impuritiesOther impurities can also interfere with ion exchange. Some of them are listed below with their effect and possible remedies.
|
||||||||||||||||||||||||||||||||||||||||
Amberpack, Upcore, ADI & ADN are trademarks of DuPont |
||||||||||||||||||||||||||||||||||||||||

Top