Ion exchange resins applications
A general overview
Contents
1.1. Softening
1.2. Dealkalisation
1.3. Demineralisation
1.4. Mixed bed polishing
1.5. Condensate polishing
1.6. Ultrapure water
1.7. Drinking water
2.1. Softening of water used for sugar extraction
2.2. Softening of sugar juices before evaporation
2.3. The NRS softening process
2.4. The Gryllus softening process
2.5. Demineralisation of sugar juices before evaporation
2.6. Colour removal from sugar syrups after evaporation
2.7. The Quentin process
2.8. Sugar recovery from molasses
2.9. Sucrose inversion
2.10. Chromatographic separation
2.11. Glucose treatment
3. Other applications in the food industry
3.1. Dairy products
3.2. Beverages
3.3. Fruit juices
3.4. Recovery of polyphenols
3.5. Citric acid
3.6. Aminoacids
3.7. Sorbitol demineralisation
3.8. Gelatine demineralisation
4. Applications in the chemical industry
4.1. Recovery or removal of metals
4.2. Caustic soda and chlorine production
4.3. Phenol
4.4. Hydrogen peroxide purification
4.5. Removal of aldehydes
4.6. Selective removal of various elements
5.1. Alkylation
5.2. Condensation
5.3. Esterification
5.4. Etherification
5.5. Dehydration
5.6. Hydrogenation
6.1. Extraction and purification of antibiotics
6.2. Slow-release formulations
6.3. Resins used as drugs
6.4. Taste-masking
6.5. Production chromatography
7.1. Mining industry
7.2. Enzyme immobilisation
7.3. Hydroculture
7.4. Oil removal by coalescence
1. Applications of ion exchange resins in water treatment
Water softening and demineralisation are also described with chemical reactions in the IX basics page. And regeneration methods are in another page.
 1.1. Softening
1.1. Softening
A strongly acidic cation exchange resin is used here in the sodium form. The ions forming hardness, essentially calcium and magnesium, are exchanged for the sodium ions of the resin, and the softened water can be used for several purposes:
- Laundries
- Domestic water boilers
- Low pressure industrial boilers
- Textile
- AmberliteTM IR120 Na, AmberjetTM 1000 Na
- Amberlite SR1L Na for drinking water
Residual hardness < 0.02 meq/L (1 mg/L as CaCO3) with reverse flow regeneration
Regeneration: brine (NaCl as a 10 % solution)
1.2. De-alkalisation
In a water containing bicarbonates — most waters in Western and Central Europe do — calcium and magnesium associated with bicarbonate ions are exchanged for hydrogen ions from a weakly acidic cation exchange resin. This is called removal of temporary hardness. The treated water contains carbon dioxide that can be removed with a degasifier. The salinity of the treated water is lower than that of the feed water. Dealkalisation is used:
- To treat water used to make beverages in breweries and soft drink plants
- To soften drinking water supplies in municipalities
- At home, to filter, soften and partially demineralise the water you use to make tea or coffee
- As a first demineralisation step
- For certain industrial processes
- Amberlite IRC86 for industrial water
- Amberlite PWC13 for municipal drinking water
- ImacTM HP333 and HP335 for household filter cartridges
Residual alkalinity = very low (endpoint at 10 % of the raw water alkalinity)
Residual hardness = permanent hardness (TH – Alk)
Regeneration : Acid (preferably HCl at 5 % concentration)
1.3. Demineralisation
All ions must be removed from water. Therefore the water passes first through cation exchange resins in the hydrogen form, then through anion exchange resins in the hydroxyl or free base form. All cations are replaced by ions from the cation resin, and all anions for the ions of the anion resin. These H+ and OH— ions recombine to create new water molecules (H2O). The treated water contains only traces of sodium and silica.
Resins used:
- Amberlite IRC86 (weakly acidic resin)
- Amberlite IR120 or Amberjet 1000 (strongly acidic resin)
- Amberlite IRA96 or IRA67 (weakly basic resin)
- Amberlite IRA402 or Amberjet 4200 or 4600 (strongly basic resin)
Treated water quality
Conductivity: 0.2 to 1 µS/cm with reverse flow regeneration
Residual silica 5 to 50 µg/L depending on the silica concentration in the feed water and the quantity of caustic regenerant.
These values are lower than those obtained with other technologies, such as reverse osmosis or distillation.
Note that the pH value should not be used as a process control, as it is impossible to measure the pH of a water with less than say 5 µS/cm conductivity.
Regeneration
Cation exchange resins: strong acid (HCl or H2SO4)
Anion exchange resins: caustic soda (NaOH)
1.4. Mixed bed units
1.4.1. Polishing mixed beds
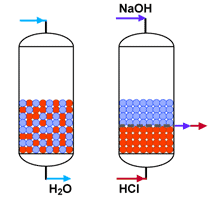 When an even better treated water quality is required, close to that of totally pure water, a polishing vessel is installed after a primary demineralisation plant. It is filled with cation and anion exchange resins, which must be mixed during the loading run, but separated for regeneration. The separation is carried out with an upflow backwash step, and requires resins with appropriate particle sizes and densities.
When an even better treated water quality is required, close to that of totally pure water, a polishing vessel is installed after a primary demineralisation plant. It is filled with cation and anion exchange resins, which must be mixed during the loading run, but separated for regeneration. The separation is carried out with an upflow backwash step, and requires resins with appropriate particle sizes and densities.
Resins used:
- Amberjet 1000 or 1500 (strongly acidic resin)
- Amberjet 4200 or 4400 (strongly basic resin)
Treated water quality
Conductivity: 0.055 to 0.1 µS/cm
Residual silica: 1 to 10 µg/L.
Note that the pH value should not be used as a process control, as pH meters are unable to operate at 1 µS/cm conductivity or below.
Regeneration
Cation exchange resins: strong acid (HCl or H2SO4)
Anion exchange resins: caustic soda (NaOH)
1.4.2. Working mixed beds
For low salinity waters, or when only moderate demineralised water volumes are required, mixed bed units can be installed and fed directly with city water or reverse osmosis permeate. These units are called "Working MBs". The resins used are essentially the same as those for polishing mixed bed units. A special case is Service De-Ionisation (SDI) — using mixed bed columns or cartridges regenerated off-site — described in a separate page.
 1.5. Condensate polishing
1.5. Condensate polishing
Steam boilers in nuclear and fossil-fuelled power stations require extremely pure demineralised water to prevent deposits on the turbine blades and avoid corrosion in the steam circuit. In many cases, the condensate circuit is fitted with mixed beds. The units are operated at a high specific flow rate and the resins must be very tough. See details in the condensate polishing page.
![]() 1.6. Ultrapure water
1.6. Ultrapure water
In the production of integrated circuits, semiconductor chips and liquid crystal or plasma displays, water of extreme purity is required in certain processing steps. In the final mixed bed polishing unit, special resin grades with a high degree of conversion are used. See details in a separate page.
Resins used (readily mixed as supplied):
- Amberjet UP6150
- Amberjet UP6040
1.7. Drinking water
Ion exchange is a valuable technology for the selective removal or certain contaminants from underground water. See details in a separate page.
2. Use of ion exchange resins in the sugar industry
 2.1. Softening of water used for sugar extraction
2.1. Softening of water used for sugar extraction
The process it that described in point 1.1 (water softening).
2.2. Softening of beet sugar juices before evaporation
The hardness of beet sugar juices results in scaling of the heat exchanger in the evaporators. To prevent it, increase the thermal efficiency and save energy, it is usual to soften the sugar juice. The plant can then operate continuously, without frequent interruptions required for de-scaling the equipment.
In this process, the type of resin used is the same as that for water softening, but the resins must be approved for use with food and resist specific stress due to the temperature and concentration of the juice.
The calcium and magnesium ions present in the sugar juice are exchanged for sodium ions from the resin. The process is applied to thin juice, i.e. after carbonation. In general, several columns operate in parallel to ensure continuous operation.
Resins used:
- Amberlite FPC14 Na
2.3. The NRS process
This is a clever process where the resin is regenerated with a solution of caustic soda diluted in thin juice. The basic idea is that whilst calcium hydroxide is insoluble in water, the calcium ions make a soluble complex with sucrose. The spent regenerant is recycled upstream, before the carbonation step, so that the production of waste is negligible. Moreover, the juice is not diluted in water as in the traditional softening process, because the NRS process does not include sweetening-off and sweetening-on steps. The energy balance is favourable and results in steam saving.
Resins used:
- Amberlite FPC14 Na
2.4. The Gryllus process
This is an older process in which the softening resin is regenerated with thick juice, which contains high concentrations of sodium. The salt consumption is thus reduced, and again, no waste is produced, since the spent regenerant is recycled.
Resins used:
- Amberlite FPC22 Na
2.5. Demineralisation of sugar juices before evaporation
In this process, "non-sugars" are removed from thin juice to increase the efficiency of crystalllisation, i.e. the sugar yield. In general, each kilogram of removed non-sugar produces 1.4 kg of additional sugar. Otherwise, the process is similar to water demineralisation: a strongly acidic cation exchange resin and a weakly basic anion exchange resin are used, regenerated respectively with acid and caustic soda.
Resins used:
- Amberlite FPC14 Na (strongly acidic)
- Amberlite FPA53 (weakly basic)
2.6. Colour removal from cane sugar syrups after evaporation
Cane syrups usually contain many organic compounds imparting colour to the crystallised sugar and reducing the crystallisation yield. The colour removal process uses strongly basic anion exchange resins, regenerated with a sodium chloride solution. These resins are macroporous, so that high molecular mass compounds can be removed. The most efficient method uses two columns in series, the first one filled with acrylic resin, the second, polishing column with styrenic resin.
Resins used:
- Amberlite FPA98 Cl (acrylic)
- Amberlite FPA90 Cl (styrenic)
2.7. The Quentin process
Crystallisation of beet sugar is partially inhibited by the potassium and sodium ions contained in the juice, so that large quantities of sugar remain in the molasses after crystallisation. Magnesium being less "melassigenous" than sodium or potassium, the idea is to pass the thin juice through a column of strongly acidic cation exchange resin in the magnesium form. This increases the production of whit sugar and reduces the quantity of molasses.
Resins used:
- Amberlite FPC23 H (must be first converted to the Mg++ form with magnesium chloride)
2.8. Sugar recovery from molasses
This process is based on ion exclusion, a kind of ion exchange chromatography using fine mesh, uniform particle size resins. It separates sugar from non-sugars and increases the recovery of sugar contained in the molasses.
Resins used:
- Amberlite CR1220 K
2.9. Sucrose inversion
Sucrose (common sugar) is a di-saccharide. In an acidic environment, the sucrose molecule splits into two mono-saccharides: glucose and fructose, in equal proportions. Invert sugar has a more powerful sweetening power than sucrose (1.15 vs. 1.0) , and a lower tendency to crystallise, an important feature for some industrial food products. Inversion is produced by passing sugar syrup through a low cross-linked strongly acidic cation exchange resin in the H+ form.
Resins used:
- Amberlite FPC12 H
2.10. Chromatographic separation
As fructose has a higher sweetening power than glucose (1.3 vs. 0.7), invert sugar syrups can be enriched with fructose by passing the syrup through a fine mesh, very uniform strongly acidic cation exchange resin in the calcium form. As the syrup stream moves down the column, fructose moves more slowly than glucose. This results in separated bands of higher purity of each component within the column. The fructose fraction is recovered separately in view of its commercial value. The glucose fraction can be either sold as a glucose syrup, or isomerised enzymatically to produce more fructose.
Resins used:
- Amberlite CR1320 Ca
2.11. Glucose demineralisation
Glucose syrups are demineralised to increase purity. The principle is identical to that of water or sugar demineralisation. In view of the high concentration and high temperature of the syrups, resins with a good resistance to these stresses must be used.
Resins used:
- DowexTM 88 (strongly acidic resin)
- Dowex 66 (weakly basic resin)
3. Examples of other applications in the food industry
 3.1. Whey demineralisation
3.1. Whey demineralisation
Whey, a by-product of cheese production, contains valuable proteins and is used in the food industry. It is demineralised to increase purity. Again, the principle is the same as that of water or sugar demineralisation.
Resins used:
- Amberlite FPC14 (strongly acidic resin)
- Amberlite FPA51 (weakly basic resin)
3.2. Beverages
There are several applications in this area:
- Treatment of the water used to make beer or soft drinks (see chapter 1)
- De-acidification of beverages with Amberlite FPA51 (weakly basic anion resin)
- Removal of metals
- Removal of bad taste or smell
- Colour and turbidity removal with non-ionic adsorbents
3.3. Treatment of fruit juices
- Acid removal with Amberlite FPA51 (weakly basic anion resin)
- Removal of bitterness from orange juices with a non-ionic adsorbent resin, Amberlite FPX66
- Colour removal with an adsorbent resin
3.4. Recovery of polyphenols
Polyphenols are praised today for their anti-oxidant properties. They are found in many types of fruit, such as berries or red grape. Anthocyanins are polyphenols that can be recovered from grape must.
Resins used:
- Amberlite FPX68 (non-ionic adsorbent resin)
3.5. Citric acid
This acid is used as a preservative in many industrial food products. It is produced by fermentation. Its purification requires ion exchange demineralisation.
Resins used:
- Amberlite FPC22 H (strongly acidic)
- Amberlite FPA51 (weakly basic styrenic) or FPA54 (weakly basic phenolic)
3.6.Aminoacids
L-lysine and other essential aminoacids (not produced by the human body) are produced by fermentation. Lysine is recovered from the fermentation broth with a cation exchange resin in ammonium form.
Resins used:
- Amberlite FPC14 (strongly acidic)
3.7. Sorbitol demineralisation
Sorbitol is a polyol, a powerful sweetener and emollient used for instance in chewing gum. It can be produced by hydrogenation of glucose or by enzymatic processes. The final product often requires demineralisation.
Resins used:
- Amberlite FPC22 (strongly acidic)
- Amberlite FPA51 (weakly basic)
- Amberlite FPC52 and FPA90 in a polishing mixed bed
3.8. Gelatine demineralisation
Gelatine is produced from the collagen present in pig skin and bones. To produce high purity gelatine, demineralisation is required.
Resins used:
- Amberlite FPC14 or FPC22 (strongly acidic)
- Amberlite FPA53 (weakly basic acrylic)
4. Some applications in the chemical industry
 4.1. Recovery and removal of metals
4.1. Recovery and removal of metals
In surface finishing and plating shops, metals can be recovered or removed:
- Gold recovery from industrial jewelleries as cyanide complexes, with Amberlite IRA402
- Recycling of various rinse water streams in plating shops, with Amberlite 252 (for cation removal), IRA96 (for chromate), and IRA410 (for cyanide)
- Copper and iron removal from chromium plating shops with Amberlyst 15Wet
- Chromic acid recovery in plating shops with Amberlite IR120 and Amberlite IRA96
- Removal of iron from zinc baths with Amberlite IRC748
- Purification of pickling baths, removing iron and zinc as chloride complexes with Amberlite IRA402. Elution is done simply with water.
Other examples:
- Recovery of silver as a thiosulphate complex from photographic baths with Amberlite IRA67 or IRA402
- Selective mercury removal in various industries with AmbersepTM GT74, a resin with thiol functionality.
- Cadmium can be removed with the same resin
- Recovery of vanadium and copper catalysts in the production of adipic acid (a precursor of nylon) with AmberlystTM 40Wet
4.2. Production of chlorine and caustic soda
These chemicals are produced by electrolysis of saturated brine. In the production process, the absence of divalent metals is critical. A selective chelating resin is thus used to remove them (principally calcium), which reduces the initial calcium concentration from 10 – 20 mg/L down to a very low level, smaller than 20 µg/L.
Resins used:
- Amberlite IRC747 when strontium removal is not necessary
- Amberlite IRC748 when strontium must also be removed
4.3. Phenol
Two applications:
- Removal of sulphuric acid and organic acids from process streams in phenol production. A special weak base resin with a phenol-formaldehyde matrix is used.
- Removal of phenol from industrial waste. Phenol is removed on a non-ionic adsorbent resin. Regeneration is done with acetone.
Resins used:
- Amberlyst A23 for acid removal
- Amberlite XAD4 for phenol removal from waste
4.4. Hydrogen peroxide purification
Resins are used in two different processes:
- Removal of anthraquinone derivatives. These organic compounds can be removed on a non-ionic adsorbent. Regeneration is done with methanol.
- Removal of metal traces such as iron, with a strongly acidic resin. The treatment is done at a very high specific flow rate.
Resins used:
- Amberlite XAD4 for organic contaminants
- Amberlyst 15Wet for metals
4.5. Removal of aldehydes
Strongly basic anion exchange resins in the bisulfite form can remove aldehydes from various aqueous solutions. The bisulfite ion forms an addition product with the aldehyde. Here the example of formaldehyde:
R+HSO3– + HCHO —> R+HOCH2SO3–
The resin is regenerated with a 5 % sodium hydrogen sulphite solution (NaHSO3).
4.6. Selective removal of various elements
I have built up a periodic system of the elements (Mendeleev table) with brief information about the removal of several ions (mostly metals) with resins.
5. Catalysis
A catalyst is a substance that increases the rate of approach to equilibrium of a chemical reaction without being substantially consumed in the reaction.
 In the majority of processes where a mineral acid was previously used as a catalyst — notably in the petrochemical industry — a strongly acidic cation exchange resin in the H+ form is now used instead. These resin must operate under stressful conditions — often at temperatures between 130 and 170 °C — and display an acidity as high as possible.
In the majority of processes where a mineral acid was previously used as a catalyst — notably in the petrochemical industry — a strongly acidic cation exchange resin in the H+ form is now used instead. These resin must operate under stressful conditions — often at temperatures between 130 and 170 °C — and display an acidity as high as possible.
A few typical examples are shown below.
5.1. Alkylation
| Product | Octylphenol |
| Reactants | Octane + phenol |
| Catalyst | Amberlyst 15Dry |
| Temperature | 100 – 120 °C |
5.2. Condensation
| Product | Bisphenol A |
| Reactants | Acetone + phenol |
| Catalyst | Amberlyst 131 |
| Temperature | 60 – 80 °C |
5.3. Esterification
| Product | Dimethyl maleate |
| Reactants | Maleic anhydride |
| Catalyst | Amberlyst 46 |
| Temperature | 110 °C |
5.4. Etherification
| Product | Methyl-ter-butyl ether (MTBE) |
| Reactants | Isobutylene + methanol |
| Catalyst | Amberlyst 35 |
| Temperature | 40 – 80 °C |
5.5. Dehydration
| Product | Isobutylene |
| Reactant | Isobutanol |
| Catalyst | Amberlyst 35 |
| Temperature | 70 – 80 °C |
5.6. Hydrogenation
| Product | Methyl isobutyl ketone (MIBK) |
| Reactant | Acetone |
| Catalyst | Amberlyst CH28 (palladium-doped catalyst) |
| Temperature | 130 – 140 °C |
6. Pharmaceutical industry
 There are various and complex applications. As the pharmaceutical industry is intrinsically secretive, few details are known. Nevertheless, let us mention a few examples:
There are various and complex applications. As the pharmaceutical industry is intrinsically secretive, few details are known. Nevertheless, let us mention a few examples:
6.1. Extraction and purification of antibiotics
Various antibiotics use ion exchange and adsorbent resins in their production process. The objective is to purify them after extraction from fermentation broths. Examples: streptomycin, gentamycin, cephalosporin, tetracyclin.
Resins used:
- Amberlite XAD1600 (non-ionic styrenic adsorbent)
- Amberlite XAD7HP (non-ionic acrylic adsorbent)
6.2. Slow-release formulations
Powdered, highly purified ion exchange resins are used as excipients in pharmaceutical formulations. The active ingredient is adsorbed on the resin and is released more slowly in the body than it would if it were present in their original state.
Resins used:
- Amberlite IRP64 (weakly acidic)
- Amberlite IRP69 (strongly acidic)
- Amberlite IRP88 (weakly acidic in potassium form)
- DuoliteTM AP143 (strongly basic)
6.3. Resins used as drugs
The same resin types can be used as active substances in the medicine. It is obvious that they must meet very stringent specifications and be approved by health authorities. Let us mention two examples:
- Cholestyramine, a drug used to reduce the cholesterol level, is a powder based on a strongly basic anion resin in the choride form.
- Polacrilin potassium, a medicine used to regulate the potassium level in the blood, is a powder based on a weakly acidic resin with a methacrylic matrix.
- Duolite AP143 (cholestyramine)
- Amberlite IRP88 (polacrilin potassium)
6.4. Taste-masking
Similar resins are used to mask the unpleasant taste oir smell of a drug.
6.5. Production chromatography
The chromatographic separation of various molecules used as active ingredients can be done with very fine particle size resins instead of silica gels or other media.
Resins used:
- A whole range of products available as Amberchrom resins.
7. Miscellaneous applications
 7.1. Mining industry
7.1. Mining industry
The most significant application, involving thousands of cubic metres of resin, is uranium extraction. The crushed ore is treated with sulphuric acid, which brings the uranium in solution as uranium sulphate. The pregnant solution is passed through beds of strongly basic anion exchange resins, which displays a high affinity for the uranium sulphate complex.
Resins used:
- Amberjet 4400
- Ambersep 920U
7.2. Enzyme immobilisation
In enzymatic reactions, it is more convenient to immobilise the enzyme on a support rather than add it to the reaction medium. Ion exchange resins are particularly suitable for this application.
Resins used:
 AmberzymeTM oxirane
AmberzymeTM oxirane
- Duolite A568 (weakly basic resin with a phenol-formaldehyde matrix)
7.3. Hydroculture
Cation and anion exchange resins are used to retain plant nutrients in hydroponic culture: ammonium, potassium, iron, zinc (cations) as well as nitrate and phosphate (anions). Oligoelements are also loaded unto the resin. This technique allows the nutrients to be released slowly as a function of the plant requirements. An overdose of fertilizer is made impossible.
See details in another page.
Resins used:
- LewatitTM HD50
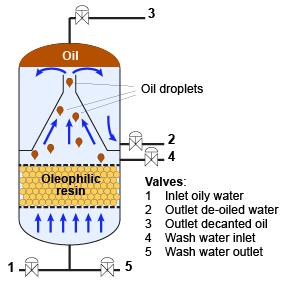 7.4. Oil removal by coalescence
7.4. Oil removal by coalescence
A special resin with oleophilic functionality is used for the removal of oil traces in condensates and other contaminated solutions. The water flows upward through the resin bed. Oil droplets are formed at the surface of the resin beads, and when they reach a critical size, they flow up to the top of the column. The resin does not require regeneration, only occasional washing.
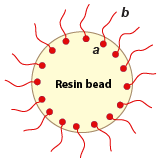
Resin used: AmberliteTM ROC110
The tail marked "b" is the oleophilic part of the functional group that is attached at the other end ("a") to the resin structure.
Trademarks
Amberjet, Amberlite, Ambersep, Amberlyst, Amberchrom, Amberzyme, Dowex, Duolite and Imac are ion exchange and adsorbent resin trademarks of Dow-DuPont.
Lewatit is a resin trademark of Lanxess.









