Ion exchange resin structure
Introduction
Ion exchange resins are polymers onto which functional groups are attached. We will examine here the chemical structure of the resin matrix, the functional groups and the overall chemical process or ion exchange resins production.The resins are produced in several steps, the two main steps being:
- Polymerisation of the resin matrix
- Functionalisation: ion exchange groups are attached to the matrix
Resin matrix
About 90 % of all ion exchange resins are based on a polystyrenic matrix. The "building block" used to make this plastic skeleton is styrene monomer, an aromatic compound also called vinylbenzene. Below are the chemical formulas: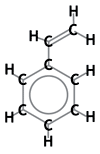 Chemical formula of styrene |
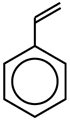 Simplified representation of styrene |
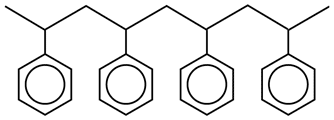
A small fraction of a polystyrene chain
The resulting linear polymer chains are entangled together, but have little physical strength: they are relatively soft, and after activation they would probably dissolve in water. To give the polymer a more stable tri-dimentional structure, the polystyrene chains are cross-linked with another molecule at the time of polymerisation. The cross-linking molecule must be able to polymerise at two or three ends. The most common cross-linker is divinylbenzene (abbreviated as DVB).
The polymerisation process is usually done in a suspension medium, either in stirred reactors (batch polymerisation) or in special "jetting" equipment. The polymers formed are very small spherical beads (200 to 500 µm in diameter). The jetting process produces very uniform bead sizes, whilst batch polymerisation results in various bead sizes with a near-Gaussian particle size distribution. See resin properties. These beads will swell to a size of 300 to 1200 µm in the subsequent functionalisation and hydration steps.
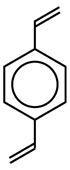 Divinylbenzene (DVB) |
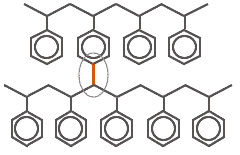 Cross-linked polystyrene |
The second bond of the DVB molecule is shown in red and attaches to the next chain of linear polystyrene. The more DVB is added to the initial reaction mixture, the more rigid is the polymer.
Most ion exchange resins are polymerised in such a way that spherical beads are obtained. This can occur either in a stirred reactor or with a jetting process. In the latter case, the bead size is very uniform.
Gel and macroporous resin structure
In the polymerisation process described above, the cross-linker is more or less evenly distributed throughout the matrix. The voids between the chains of polystyrene are called pores. They are very small and their size is only a few Å, but the size is relatively constant: the matrix has a pseudo-crystalline structure, similar to glass, and as a result the finished ion exchange resiin beads are transparent. In the picture below, the polystyrene chains are shown in blue without the aromatic chemical details, and the "bridges" formed by DVB are shown in red.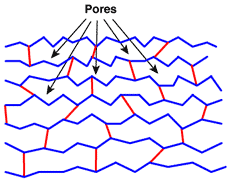
Gel structure
There is a limit to the quantity of DVB that can be used in gel type resins: too much DVB creates a stucture with very small pores, which in the final product may be a disadvantage, as larger ions cannot enter the resin beads. Additionally, highly cross-linked polymers are more difficult to activate.
To overcome this problem, macroporous resins have been invented in the 1960's. The idea is to create artifical porosity in the tri-dimensional matrix. To this effect, a third component — called porogen or phase extender — is incorporated in the reaction mixture, which does not react with the monomers, but only takes room in the system. Once the polymerisation reaction is finished, the porogen is washed out and leaves voids in the polymer structure. These are the macropores.
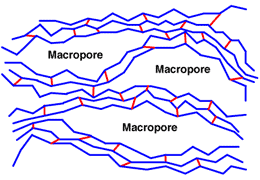
Macroporous structure
Macroporous resins have a double porosity: the small pores of the matrix itself and the large macropores created by the phase extender. The final resins are opaque. They are very stable, as the matrix is usually highly cross-linked. They also very porous, thus can exchange large ions.
Pore size
Functionalised, hydrated ion exchange resins have a pore size of approximately 1 to 2 nm (10 to 20 Å), while macroporous resins, in addition to their small gel pores, have macropores with a size of about 20 to 100 nm (200 to 1000 Å). For comparison, hydrated inorganic ions have a size of about 0.2 to 0.5 nm. Organic ions may be much bigger than that.Functionalisation of the polymer
The polymer described above must be activated to convert the plastic beads into ion exchange resins.Strongly acidic cation exchange resins
The activation is chemically simple: it is a sulphonation reaction. the polystyrene beads are contacted at high temperature with concentrated sulphuric acid. The product is a polystyrene sulphonate, which is a strong acid.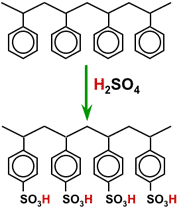
Sulphonation
After sulphonation, the resin is washed to remove excess sulphuric acid. This hydration step is a delicate operation, as it causes the resin beads to swell (functional groups are hydrated and thus grow in size). The corresponding osmotic force is considerable and can result in breaking the beads to pieces if it is not done cautiously.
This reaction produces the resin in hydrogen form. If the product is to be used as a softening resin, it must be converted in an additional step to the sodium form. This can be done with sodium carbonate, for instance.
Strongly and weakly basic anion exchange resins
The activation is more complex, as it requires two successive steps. Also, the chemicals used are more expensive, which explains that anion exchange resins are considerably more expensive than SAC resins. The first step is called chloromethylation, and is a reaction between the polymer beads and chloromethyl methylether (which is a very hazardous chemical):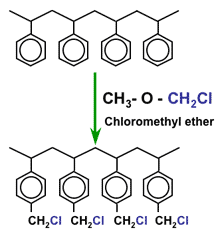
Chloromethylation
The product has chloride groups, but these are covalent, not ionised. At this stage, we don't have an anion exchange resin yet. The second step of the activation is an amination, where the covalent chloride is replaced by an amine.
Amination
The picture shows two cases of amination. The product is a quaternary ammonium chloride salt. In the first reaction, the amine used is trimethylamine. By convention, SBA resins produced with trimethylamine are called Type 1. In the second reaction, the amine is dimethylethanolamine, and the product is called Type 2. As produced, SBA resins are in the chloride (Cl—) form. They are usually stored and shipped in this form, which is more stable than the OH— form, and must be regenerated with caustic soda before use in a demineralisation system.
Regeneration
Type 1 resins are the most common strongly basic exchangers. Type 2 resins have a lower basicity than type 1. This results in a better regenerability (conversion to the OH— form) but type 2 resins are more sensitive to temperature degradation, and have a lower selectivity for ions, so that the leakage is higher in service.
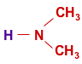
Weakly basic anion exchange resins are produced in the same way, but using a secondary instead of a tertiary amine. In most cases, dimethylamine (shown on the left) is used. The product is an ion exchange resin in the hydrochloric form, which is regenerated to the free base form for supply. This WBA resin is shown on the right. In the regenerated form, WBA resins do not have exchangeable ions. This is why they are said to be in the free base form, not in the OH form, when regenerated. They can only remove strong acids from solution, which means they must be used after decationisation. See ion exchange reactions.
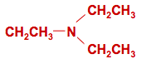 Still other amines can be used to make special anion exchange resins, such as those selective for nitrate (here on the left triethylamine), borate (N-methyl glucamine, see below), or perchlorate.
Still other amines can be used to make special anion exchange resins, such as those selective for nitrate (here on the left triethylamine), borate (N-methyl glucamine, see below), or perchlorate.
Weakly acidic cation exchange resins
These resins are not produced by activation of polystyrene beads. Instead, they are made from acrylic polymers, either starting from acrylonitrile or from methyl acrylate.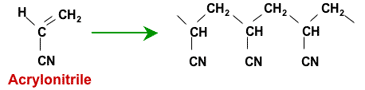
Polymerisation of acrylonitrile
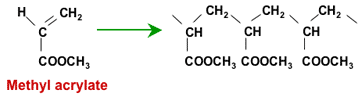
Polymerisation of methyl acrylate
The polymer obtained must be hydrolysed to form carboxylic acid groups. The polyacrylonitrile polymer is hydrolysed with sulphuric acid, whilst the polyacrylate is hydrolysed with caustic sode. In both cases, the same final compound is obtained. Here only caustic hydrolysis is shown:
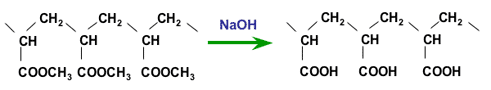
Hydrolysis of polyacrylate with caustic soda
The WAC resin product obtained is a weak acid, only partially ionised in neutral environment. Its acidity is similar to that of acetic acid. Due to the aliphatic (not aromatic) light-weight structure of the matrix, WAC resins have a higher density of active groups than resins based on polystyrene. This results in a high total ion exchange capacity.
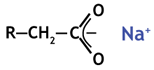
Once dissociated (with an alkaline anion, see reactions), the resin can exchange cations. Here an example with sodium. A carboxylate salt is formed.
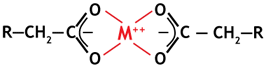
With divalent metals, a stable complex is formed between two functional groups, as shown on the left, which explains the high selectivity of WAC resins for these ions.
Acrylic anion exchange resins
WBA and SBA resins can also be made starting from an acrylic instead of an aromatic copolymer. The first polymeridsation step is the same as that for a WAC resin starting from methyl acrylate, as shown in the previous section. The polyacrylate is then reacted with a special di-amine having a primary amine at one end and a tertiary amine at the other end: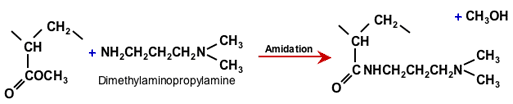
Amidation of polyacrylate with DMAPA
The product obtained is a weakly basic acrylic anion exchange resin. From this, a strongly basic resin can be obtained with an additional quaternisation step using methyl chloride or dimethyl sulphate:
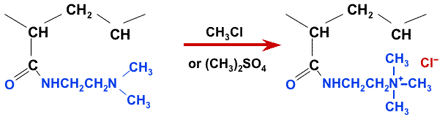
Quaternisation of an acrylic WBA to SBA
Acrylic anion resins are more hydrophilic than styrenic resins, and show a superior resistance to organic fouling.
Other functional groups
Several other resin types have been developed, mainly to produce selective resins. A few typical active groups are shown below:
Various other functional groups
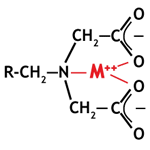 Chelating resins
Chelating resins
Most of the above special resins are of the chelating type. They are able to form complexes with metals. Their name is derived from the Greek word Χηλή meaning "claw", because the metallic ions are captured like in a claw. The picture on the right shows a metal complexed by the iminodiacetic functional group of a chelating resin. The chelating resins make complexes only with multivalent metals. These complexes are very stable. Therefore, these resins have a high selectivity and are capable of removing metals from solution preferentially. See examples of chelating resins in the resin types page, with the corresponding applications.
A general summary of the most common resins and their active groups is shown in the "resin types" page.
See also the table of all known ion exchange resins, past and present.
Amberlite, Ambersep, Dowex, Duolite, and Imac are trademarks of DuPont. Lewatit is a trademark of Lanxess, Diaion is a trademark of Mitsubishi Chemicals.








